Criegee, R. Oxidation with quadrivalent lead salts. II. Oxidative cleavage of glycols. Ber. 1931, 64B, 260-266.
Criegee, R., et al. Newer Methods of Preparative Organic Chemistry (Interscience Publishers, New York, 1948) 657
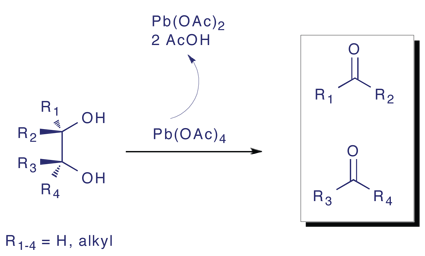
The Criegee oxidation allows diols to be cleaved with good yield under mild conditions in the presence of lead tetraacetate. This is a complementary method to ozonolysis of double bonds which are easily dihydroxylated in the presence of osmium tetroxide. A number of additional functional groups have been shown to cleave in a similar manner upon treatment with lead tetraacetate these include β-amino alcohols, 1,2-diamines, α-hydroxy aldehydes and ketones, α-diketones and α-keto aldehydes.

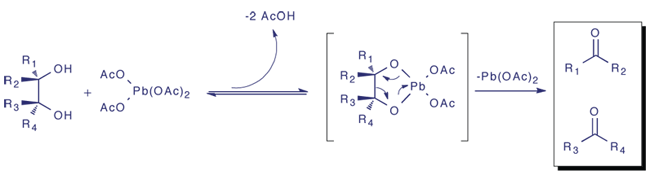
1) Formation of 5 membered bidentate 1,2glycol Pb complex.
2) Breakdown of the 5 membered intermediate via 2 electron process as Pb(IV) abstracts the bonde electron pair of the oxygen and is reduced to Pb(II).
3) Addition of acetic acid slows the reaction significantly by shifting equilibrium to the left.
4) In the case where the intermediate 5 membered ring can not form such as for bicyclic trans diols a concerted electron process has been proposed involving a third acetate group attached to the metal.